What is titanium dioxide?
Titanium dioxide is a harmless substance used in everyday life. It is part of chewing gum, white paints, topcoat in the paper industry, filler in the rubber and plastics industry. Its utilization rate is well illustrated by the global production of TiO2, which is millions of tonnes every year and growing. It is no exaggeration to say that it is part of our daily lives, that we are surrounded by it, even if we do not know it. The reason why it is widely used in the paint industry is that although the large crystals of TiO2 (rutile) are transparent, their fine grains scatter light so much that they can make films of high opacity (light transmission) (N.N. Greenwood, A. Earnshaw, Chemistry of Elements, 2004). Thanks to this feature, the ultra-fine (20-50 nm) TiO2 has been found to be a UV filter for use in skin protection and cosmetics.
Thanks to the development of chemical synthesis methods, TiO2 nanoparticles of smaller diameter have been made available over time, and their antimicrobial effect has been revealed almost immediately.
Why nano?
Today’s accepted definition of nanoparticles is particles smaller than 100 nanometres. There are other definitions also available, so you can see 20, 50, etc. nanometre limits as well in literature. In the past, these particles have been studied by the science of colloids, while nowadays we are talking about nanotechnological achievements. It is an extremely intensively evolving field, as illustrated by the fact that while in 2000 some tens of patents were created using nanotechnology, five years later thousands of patents were recorded. Numerous compounds can be made into nanoparticles, even unintentionally. A simple example is cigarette smoke, which also contains nanoparticles..
Anyone less familiar with nanotechnology would be surprised to learn how much the nanoworld is part of our daily lives. Among other things, a significant proportion of titanium dioxide in chewing gum is a nanoparticle, nano-silica is used as an anti-caking agent, nano-droplets enhance the taste of cocoa, etc.; the list is very long. The crucial feature of nanoparticles is that their surface area is large in relation to their volume. While one gram of material is a few square centimetres, depending on its shape, one gram of nanoparticles of the same material can be hundreds of square meters. As a result, surface-specific features are brought to the forefront, creating new features.
TiO2 nanoparticles
water by light. Their study provided basis for the development that eventually led to the birth of TIOXtm. Research quickly pointed out that light and water are required for TiO2 nanoparticles to function. This phenomenon, called heterogeneous photocatalysis, involves the semiconductor absorbing light of a sufficient wavelength into a higher energy state that can be transmitted to another matter in close proximity. TiO2 has a semiconductor valence band and a conductive band, the former occupies electrons, and the latter is empty. When a beam of energy greater than the energy difference of the two bands illuminates the semiconductor, the electrons are excited to the conductive band, leaving an empty “positive hole” behind them.
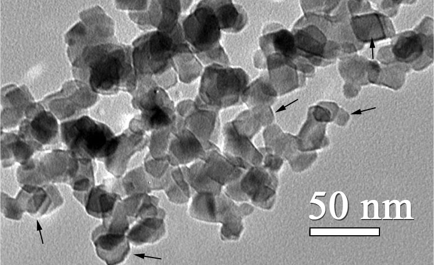 This is an unstable condition that can be resolved in many ways. Most often, the electron simply jumps back into the valence band while emitting the absorbed light. If there is (are) compound(s) nearby for electron transfer and electron capture, the electron-hole pair may also be terminated by the compound(s). In this case, the semiconductor will be in a ground state while the material interacting with it will be excited, unstable. It is most commonly accepted that oxygen on the surface forms a superoxide radical by electron uptake, whereas positive holes are eliminated by the formation of hydroxyl radicals in water.
This is an unstable condition that can be resolved in many ways. Most often, the electron simply jumps back into the valence band while emitting the absorbed light. If there is (are) compound(s) nearby for electron transfer and electron capture, the electron-hole pair may also be terminated by the compound(s). In this case, the semiconductor will be in a ground state while the material interacting with it will be excited, unstable. It is most commonly accepted that oxygen on the surface forms a superoxide radical by electron uptake, whereas positive holes are eliminated by the formation of hydroxyl radicals in water.
The resulting compounds react with the microorganisms on the surface and destroy their membrane, resulting in an antimicrobial effect. In summary, by absorbing light, the nanoparticle creates a condition in its microenvironment that interferes with the organisms on its surface, thereby inhibiting their reproduction and deteriorating their living conditions. The problem that had not been solved for a long time is that titanium dioxide can only be excited by UV light, which is a component that is insufficient in sunlight, and almost non-existent in most artificial light sources. As a result of intensive research, we are now able to tune the excitation wavelength to a certain extent, which has opened the way to everyday use.
While early solutions required high-intensity UV light, we have now succeeded in adapting it to the light emitted by everyday lamps and the light of the sun. This required a range of innovative solutions and this is what TIOXtm offers: a consciously made cocktail of nanoparticles of different sizes that, unlike most products on the market, works well with traditional and new types of light sources. For a long time, the development of a sufficiently stable layer has also been a problem. Although the antimicrobial action of TiO2 nanoparticles has been known for some time, it has been difficult to provide a durable, mechanical stress resistant surface that can meet the requirements of everyday use. As a result of our developments, we have overcome this problem and we offer a solution that will not lose its effectiveness even when exposed to the challenges of daily living, which mean a huge challenge for the stability of nanostructured surfaces.
 For this, we use the so-called sol-gel method. The carrier material of the applied nanoparticulate suspension forms chemical bonds under the influence of air and forms a very thin two-dimensional stable film that is invisible to the eye. The substrate is partly bound to the surface and to itself through oxygen bridges and partly through so-called secondary chemical bonding forces (Van der Walls forces), in which silica (a constituent of quartz, glass, sand) plays an important role. The precursor of the surface-forming compound is liquid and therefore it fills the tiny sub microscopic gaps of the object to be treated and clings to it similarly to the roots of trees. The nanoparticles in suspension are also embedded in the film in this way: due to their huge surface area, these secondary bonding forces are added together and eventually become so large that the binding stability of the particles goes well beyond the requirements of everyday use. The surface is formed using a low-pressure atomizer which provides complete coverage and homogeneous distribution.
For this, we use the so-called sol-gel method. The carrier material of the applied nanoparticulate suspension forms chemical bonds under the influence of air and forms a very thin two-dimensional stable film that is invisible to the eye. The substrate is partly bound to the surface and to itself through oxygen bridges and partly through so-called secondary chemical bonding forces (Van der Walls forces), in which silica (a constituent of quartz, glass, sand) plays an important role. The precursor of the surface-forming compound is liquid and therefore it fills the tiny sub microscopic gaps of the object to be treated and clings to it similarly to the roots of trees. The nanoparticles in suspension are also embedded in the film in this way: due to their huge surface area, these secondary bonding forces are added together and eventually become so large that the binding stability of the particles goes well beyond the requirements of everyday use. The surface is formed using a low-pressure atomizer which provides complete coverage and homogeneous distribution.